
Protein-Membrane Research
Antimicrobial Peptides
Antimicrobial resistance (AMR) is one of today’s most urgent public health challenges, threatening to render many current treatments ineffective. In 2019 alone, antibiotic-resistant infections claimed around 5 million lives, surpassing mortality rates for HIV and malaria and placing immense strain on healthcare systems worldwide. Antimicrobial peptides represent a promising class of compounds for developing novel treatments. By precisely tuning their properties, these peptides can selectively target and eliminate bacteria, viruses, or even cancer cells through mechanisms that involve bypassing or disrupting cellular membranes. Using multiscale computer simulations, we systematically investigate the intricate interplay between peptides and lipids to identify critical molecular features—such as amphiphilicity, flexibility, and length—that underlie peptide activity. Our computational designs are verified in-house in our BSL-2 laboratory by a dedicated staff team. We are advancing our de novo designed pore-forming peptides, which have shown promising antimicrobial activity in mouse models, and developing cell-penetrating peptides for cargo delivery. The molecular insights gained support the rational design of new antimicrobial peptides with applications in medicine, cosmetics, and disinfection.

Curvature Sensing and Modulating Proteins
Certain proteins have evolved to sense and respond to membrane curvature, allowing them to self-organize within specific regions of cellular membranes. This capability is crucial for processes such as endocytosis, exocytosis, cell division, and signaling. However, viruses can exploit this protein colocalization and membrane architecture to hijack cellular machinery, promoting their budding, infection, and replication. Despite the importance of membrane curvature, our understanding of protein localization at curved membranes remains limited due to the complex interplay between protein shape and sequence, and membrane curvature and composition. Building on our previous research, we design peptides that can sense positive or negative membrane curvature and remodel membranes by inducing fusion or pore formation. Our primary objective is to advance these peptide sequences to specifically target the human plasma membrane and precise membrane curvatures, with potential applications in biotechnology and therapy.
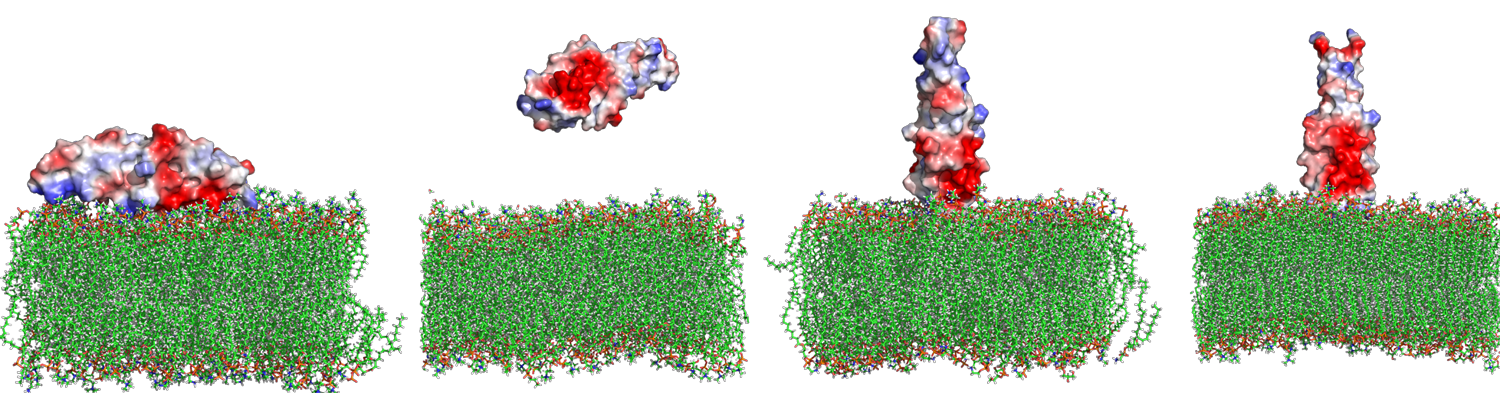
Fusogenic Peptides
Membrane fusion is an essential biological process that plays a crucial role in neurotransmission, intracellular trafficking, immune responses, and viral infections. Despite its fundamental biological significance and potential applications in drug delivery, the molecular understanding of membrane fusion and its control remains elusive. Our research focuses on designing novel fusogenic peptides for use in liposomal and lipid nanoparticle drug formulations. Building upon our expertise in membrane-active peptides and de novo peptide design, we develop computational approaches to study the critical steps of membrane fusion: lipid protrusions, formation of the membrane stalk and hemifusion diaphragm, and the opening of the fusion pore. Additionally, we have access to a fully equipped laboratory with dedicated staff to perform advanced biophysical assays, allowing us to experimentally test our computational designs. Ultimately, we aim to design peptides that induce membrane fusion, paving the way for targeted drug delivery and enhanced therapeutic efficacy in clinical applications.

